
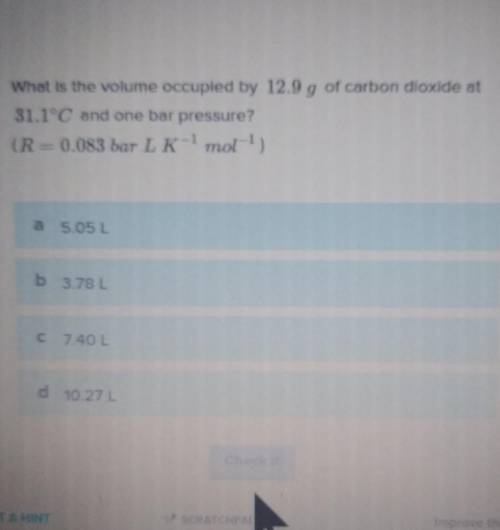
*free 16 pts boom*ONLY A TRUE MORAL GOD CAN ANSWER THIS. LESS THAN ONE PERCENT CAN ANSWER THIS SHI* THIS QUESTION WONT LET ME PASS IF I DONT GET IT RIGHT What is the volume occupied by 12.9 g of carbon dioxide at
311°C and one bar pressure?
so basically ideal gas laws formula PV= nRT and solve for V, I'm worn out someone PLEASE do it, last question and I'm done!!
Jesus... is that you?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, Queenquestion5967
When the following equation is balanced using the smallest possible integers, what is the coefficent of oxygen gas? c7h16o(g) + o2(g) → co2(g) + h2o(g) -1 -5 -8 -16 -21
Answers: 3

Chemistry, 22.06.2019 10:30, Riplilpeep
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 13:00, nadikadiaz1
These questions are based on the attached photo. the experiment is about burning magnesium metal with oxygen. 1. write the balanced chemical equation for the reaction you are performing. 2. calculate the mass of magnesium metal used in each trial. o trial 1: o trial 2: 3. calculate the actual yield of magnesium oxide for each trial. o trial 1: o trial 2: 4. magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. o trial 1: o trial 2: 5. determine the percent yield of mgo for your experiment for each trial. o trial 1: o trial 2: 6. determine the average percent yield of mgo for the two trials. your company currently uses a process with a similar cost of materials that has an average percent yield of 91 percent. if the average percent yield of this process is higher than that, this could save the company money. what is your recommendation to the company? support your recommendation using your data, calculations, and understanding of stoichiometry gathered from this lab.
Answers: 1
Do you know the correct answer?
*free 16 pts boom*ONLY A TRUE MORAL GOD CAN ANSWER THIS. LESS THAN ONE PERCENT CAN ANSWER THIS SHI*...
Questions in other subjects:

Physics, 03.10.2019 08:50

Physics, 03.10.2019 08:50

History, 03.10.2019 08:50


Mathematics, 03.10.2019 08:50

Health, 03.10.2019 08:50

Biology, 03.10.2019 08:50

Health, 03.10.2019 08:50


English, 03.10.2019 08:50






