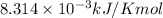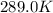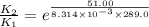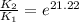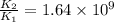Chemistry, 01.04.2020 22:23, milkshakegrande101
The presence of a catalyst provides a reaction pathway in which the activation energy of a reaction is reduced by 51.00 kJ ⋅ mol − 1 51.00 kJ⋅mol−1 . Uncatalyzed: A ⟶ B A⟶B E a = 136.00 kJ ⋅ mol − 1 Ea=136.00 kJ⋅mol−1 Catalyzed: A ⟶ B A⟶B E a = 85.00 k J ⋅ mol − 1 Ea=85.00 kJ⋅mol−1

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:50, ShlomoShekelstein
Why do scientists look for patterns in the world? a. patterns can explain observations. b. patterns never change, no matter what. c. patterns are easy for scientists to detect. d. patterns are all the same, through all time.
Answers: 1


Chemistry, 23.06.2019 02:50, igraha17
Dumbledore decides to gives a surprise demonstration. he starts with a hydrate of na2co3 which has a mass of 4.31 g before heating. after he heats it he finds the mass of the anhydrous compound is found to be 3.22 g. he asks everyone in class to determine the integer x in the hydrate: na2co3·xh2o; you should do this also. round your answer to the nearest integ
Answers: 2
Do you know the correct answer?
The presence of a catalyst provides a reaction pathway in which the activation energy of a reaction...
Questions in other subjects:










English, 10.09.2019 18:10


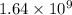
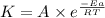
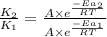
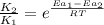
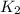 = rate of reaction with catalyst
= rate of reaction with catalyst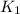 = rate of reaction without catalyst
= rate of reaction without catalyst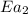 = activation energy with catalyst
= activation energy with catalyst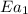 = activation energy without catalyst
= activation energy without catalyst