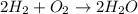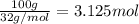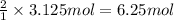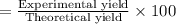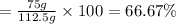Chemistry, 01.04.2020 21:45, mariarodriguezout9cj
Consider the chemical equation for the production of water: 2 H2+O2→2 H2O. If 100 grams of oxygen gas are used, what would the percent yield be if 75 g of H2O was produced? Show your work.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:10, ChloeLiz7111
Nitrogen (n), phosphorus (p), and potassium (k) are the main nutrients in plant fertilizers. according to an industry convention, the numbers on the label refer to the mass percents of n, p2o5, and k2o, in that order. calculate the n: p: k ratio of a 30: 10: 10 fertilizer in terms of moles of each element, and express it as x: y: 1.0.
Answers: 1

Chemistry, 22.06.2019 13:00, nauticatyson9
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Do you know the correct answer?
Consider the chemical equation for the production of water: 2 H2+O2→2 H2O. If 100 grams of oxygen ga...
Questions in other subjects:





Mathematics, 31.01.2020 18:02



Biology, 31.01.2020 18:03