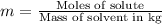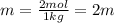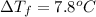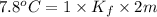Chemistry, 01.04.2020 18:36, kwarwick0915
Two moles of a nonelectrolyte solute are dissolved in 1 kg of an unknown solvent. the solution freezes at 7.8°c below its normal freezing point. what is the molal freezing-point constant of the unknown solvent? suggest a possible identity of the solvent.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:20, greenbyron88
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 14:30, davidrodriguez122001
Which of the following describes a situation where competition between producers exists
Answers: 1

Do you know the correct answer?
Two moles of a nonelectrolyte solute are dissolved in 1 kg of an unknown solvent. the solution freez...
Questions in other subjects:

Biology, 10.04.2020 00:59



Social Studies, 10.04.2020 00:59

Mathematics, 10.04.2020 00:59



History, 10.04.2020 01:00



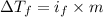
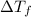 =depression in freezing point =
=depression in freezing point = 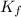 = freezing point constant
= freezing point constant