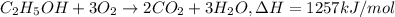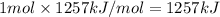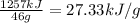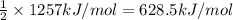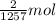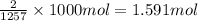Chemistry, 01.04.2020 17:26, KariSupreme
Reaction enthalpy for ethanol oxidation, C2H5OH+3O2 -> 2CO2 + 3H2O is 1257 kJ/mole. Energy content per mol fuel kJ Energy content per gram fuel = kJ Energy released per mol CO2 formed kJ Energy released per mol O2 consumed= kJ Moles of CO2 formed per 1000 kJ energy released

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, jasminortega2002
Which of the following compounds does not contain molecules? question 2 options: co2 h2 nacl h2o
Answers: 1

Chemistry, 21.06.2019 22:30, ayoismeisalex
Which of these sequences lists the correct order for the creation of sedimentary rock from sediment? a. deposition, burial, compaction, cementation b. burial, deposition, compaction, cementation c. compaction, deposition, burial, cementation d. cementation, deposition, burial, compaction
Answers: 1

Chemistry, 22.06.2019 08:30, omoaye
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 12:00, BakerElsie02
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1
Do you know the correct answer?
Reaction enthalpy for ethanol oxidation, C2H5OH+3O2 -> 2CO2 + 3H2O is 1257 kJ/mole. Energy conten...
Questions in other subjects:


Chemistry, 03.02.2020 17:03


History, 03.02.2020 17:03


Mathematics, 03.02.2020 17:03



Social Studies, 03.02.2020 17:03

Mathematics, 03.02.2020 17:03