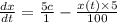Chemistry, 01.04.2020 16:06, tankhill5534
Fish tank initially contains 35 liters of pure water. Brine of constant, but unknown, concentration of salt is flowing in at 5 liters per minute. The solution is mixed well and drained at 5 liters per minute. Let xx be the amount of salt, in grams, in the fish tank after tt minutes have elapsed. Find a formula for the rate of change in the amount of salt, dx/dtdx/dt, in terms of the amount of salt in the solution

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:10, andybiersack154
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 06:30, yolo123321
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 08:00, stephstewart1209
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 23:00, lilsnsbsbs
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2
Do you know the correct answer?
Fish tank initially contains 35 liters of pure water. Brine of constant, but unknown, concentration...
Questions in other subjects:

Mathematics, 15.11.2019 05:31

English, 15.11.2019 05:31

English, 15.11.2019 05:31


Mathematics, 15.11.2019 05:31


Mathematics, 15.11.2019 05:31

Mathematics, 15.11.2019 05:31


English, 15.11.2019 05:31


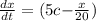

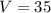 lit
lit
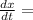 ( Rate of salt enters tank - rate of sat leaves tank )
( Rate of salt enters tank - rate of sat leaves tank )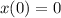
 ,
,