
Chemistry, 01.04.2020 05:41, ashtonbillups
At 25 oC, hydrogen iodide breaks down very slowly to hydrogen gas and iodine vapor with a rate constant of 2.4 x 10-21L/mol. s. If 0.0100 mol of HI(g) is placed into a 1.0 L container at 25 oC, how long will it take for the concentration of HI to reach 0.00900 mol/L?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, electrofy456
What diagram shows the ionic compound of magnesium oxide
Answers: 2

Chemistry, 22.06.2019 08:00, juliannxkim
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 08:30, itzhari101
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1
Do you know the correct answer?
At 25 oC, hydrogen iodide breaks down very slowly to hydrogen gas and iodine vapor with a rate const...
Questions in other subjects:





English, 16.12.2020 18:10


Mathematics, 16.12.2020 18:10

Mathematics, 16.12.2020 18:10

Mathematics, 16.12.2020 18:10

Health, 16.12.2020 18:10


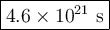
![\dfrac{1}{\text{[A]}} =\dfrac{1}{\text{[A]}_{0}}+ kt](/tpl/images/0575/0646/44bc3.png)
![\begin{array}{rcl}\dfrac{1}{\text{[A]}} & = & \dfrac{1}{\text{[A]}_{0}}+ kt\\\\\dfrac{1}{0.00900 }& = & \dfrac{1}{0.0100} + 2.4 \times 10^{-21} \, t\\\\111.1&=& 100.0 + 2.4 \times 10^{-21} \, t\\\\11.1& = & 2.4 \times 10^{-21} \, t\\t & = & \dfrac{11.1}{ 2.4 \times 10^{-21}}\\\\& = & \mathbf{4.6 \times 10^{21}}\textbf{ s}\\\end{array}\\\text{It will take $\large \boxed{\mathbf{4.6 \times 10^{21}}\textbf{ s}}$ for the HI to decompose}](/tpl/images/0575/0646/4ef33.png)




