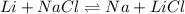For the chemical reaction, Li + NaCl <-- --> Na + LiCl, the rate law would be...
A...

Chemistry, 01.04.2020 01:11, sammybrain
For the chemical reaction, Li + NaCl <-- --> Na + LiCl, the rate law would be...
A. Rate = k[Li][NaCl]
B. Rate = k[Na] [LiCl]
C. Rate = k[Li] + [NaCl]
D. Rate = k[Na] + [LiCl]

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, applejulianamoreno
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3


Chemistry, 23.06.2019 00:30, tateandvioletAHS14AY
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1

Chemistry, 23.06.2019 05:00, kayranicole1
How is electrolysis most commonly used to produce an energy source? a - splitting water molecules produces oxygen, which organisms breathe to fuel their bodies. b - splitting water molecules produces hydrogen gas, which is used to power machines through hydrogen fuel cells. c - splitting carbon dioxide molecules produces coal, a form of carbon that can be burned to produce heat. d - splitting carbon dioxide molecules produces natural gas, which can be burned to generate electricity in power plants.
Answers: 1
Do you know the correct answer?
Questions in other subjects:




Mathematics, 23.03.2020 21:16

Biology, 23.03.2020 21:16


Biology, 23.03.2020 21:17

Social Studies, 23.03.2020 21:17


Mathematics, 23.03.2020 21:17


![\text{Rate}=k[Li][NaCl]](/tpl/images/0574/5230/1bc48.png)