HELP PLEASE
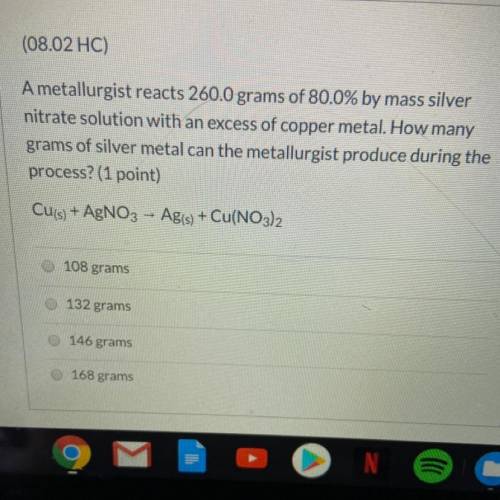
A metallurgist reacts 260.0 grams of 80.0% by mass silver nitrate solution w...

Chemistry, 31.03.2020 18:56, sparkyjones02
HELP PLEASE
A metallurgist reacts 260.0 grams of 80.0% by mass silver nitrate solution with an excess of copper metal. How many grams of silver metal can the metallurgist produce during the process?
Cu(s) + AgNO3 - Ag(s) + Cu(NO3)2
Answers
A.108 grams
B.132 grams
C.146 grams
D.168 grams

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:20, JKINGblackstar3502
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1

Do you know the correct answer?
Questions in other subjects:



Social Studies, 30.03.2021 01:00



Mathematics, 30.03.2021 01:00

Mathematics, 30.03.2021 01:00



Social Studies, 30.03.2021 01:00






