

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:50, dootdootkazoot
Aluminum–lithium (al-li) alloys have been developed by the aircraft industry to reduce the weight and improve the performance of its aircraft. a commercial aircraft skin material having a density of 2.47 g/cm3 is desired. compute the concentration of li (in wt%) that is required.
Answers: 3


Chemistry, 22.06.2019 07:00, uniqueray33
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 09:00, Ezekielcassese
What is the percentage composition of carbon in the compound ch4
Answers: 1
Do you know the correct answer?
The natural distribution of the isotopes of a hypothetical element is 60.795% at a mass of 281.99481...
Questions in other subjects:

Mathematics, 03.03.2021 06:30


Biology, 03.03.2021 06:30

Chemistry, 03.03.2021 06:30


Mathematics, 03.03.2021 06:30

Social Studies, 03.03.2021 06:30


Chemistry, 03.03.2021 06:30

Social Studies, 03.03.2021 06:30


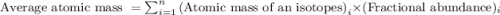 .....(1)
.....(1)![\text{Average atomic mass of element}=[(281.99481\times 0.60795)+(283.99570\times 0.22122)+(286.99423\times 0.17083)]\\\\\text{Average atomic mass of element}=283.291amu](/tpl/images/0572/8936/1f7da.png)




