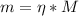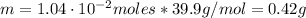Chemistry, 31.03.2020 04:40, Gogontle8347
A chemist must prepare 800.0mL of sodium hydroxide solution with a pH of 12.10 at 25°C. She will do this in three steps: Fill a 800.0mL volumetric flask about halfway with distilled water. Weigh out a small amount of solid sodium hydroxide and add it to the flask. Fill the flask to the mark with distilled water. Calculate the mass of sodium hydroxide that the chemist must weigh out in the second step. Round your answer to 2 significant digits.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:30, sheazy3709
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2

Chemistry, 23.06.2019 00:30, hdhshshs741
An unknown insoluble substance displaced the water shown. it's mass is indicated on the triple beam balance. mass = a. 694 b. 693.5 c. 693.0 d.693.8
Answers: 1


Chemistry, 23.06.2019 13:30, leeleelynn
If a fast moving car making a loud noise approaches and moves past the person what will happen as the distance between the two increases?
Answers: 1
Do you know the correct answer?
A chemist must prepare 800.0mL of sodium hydroxide solution with a pH of 12.10 at 25°C. She will do...
Questions in other subjects:

Mathematics, 25.07.2019 20:10





Mathematics, 25.07.2019 20:10

History, 25.07.2019 20:10




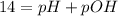

![pOH = -log ([OH^{-}])](/tpl/images/0572/8536/5638e.png)
![[OH]^{-} = 10^{-pOH} = 10^{-1.90} = 0.013 M](/tpl/images/0572/8536/7c501.png)
![\eta = ([OH]^{-})*V = 0.013 mol/L * 0.800 L = 1.04 \cdot 10^{-2} moles](/tpl/images/0572/8536/c1790.png)