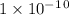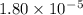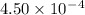Chemistry, 31.03.2020 04:19, jessicaflower277
Given the following acids and their Ka values: Phenol, C6H5OH Ka = 1.00 × 10-10 Acetic acid, CH3CO2H Ka = 1.80 × 10-5 Nitrous acid, HNO2 Ka = 4.50 × 10-4 What is the order of increasing base strength for C6H5O−, CH3COO−, and NO2−? Group of answer choices

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 15:30, lizzyhearts
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3


Do you know the correct answer?
Given the following acids and their Ka values: Phenol, C6H5OH Ka = 1.00 × 10-10 Acetic acid, CH3CO2H...
Questions in other subjects:

Mathematics, 13.12.2020 02:50

Computers and Technology, 13.12.2020 02:50


Arts, 13.12.2020 02:50

Mathematics, 13.12.2020 02:50




Biology, 13.12.2020 02:50


 <
<  <
< 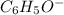
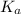 value is a measure of acidic strength. The larger the
value is a measure of acidic strength. The larger the