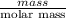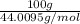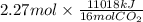Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, jetblackcap
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1


Do you know the correct answer?
Using the following equation for the combustion of octane, calculate the heat associated with the fo...
Questions in other subjects:











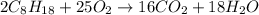 ;
; 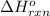 = -11018 kJ
= -11018 kJ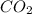 = 100 g
= 100 g