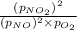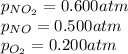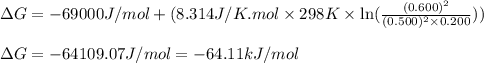For a gaseous reaction, standard conditions are 298 K and a partial pressure of 1 atm for all species. For the reaction 2 NO ( g ) + O 2 ( g ) − ⇀ ↽ − 2 NO 2 ( g ) the standard change in Gibbs free energy is Δ G ° = − 69.0 kJ/mol . What is ΔG for this reaction at 298 K when the partial pressures are P NO = 0.500 atm , P O 2 = 0.200 atm , and P NO 2 = 0.600 atm ?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, tddreviews
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1


Chemistry, 22.06.2019 20:50, iluminatioffial9699
One nanometer is equal to how many meters?
Answers: 2
Do you know the correct answer?
For a gaseous reaction, standard conditions are 298 K and a partial pressure of 1 atm for all specie...
Questions in other subjects:

Engineering, 16.02.2020 20:46




Mathematics, 16.02.2020 20:48

History, 16.02.2020 20:48

Mathematics, 16.02.2020 20:48


Mathematics, 16.02.2020 20:48


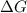 for the given reaction at 298 K is -64.11 kJ/mol
for the given reaction at 298 K is -64.11 kJ/mol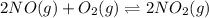
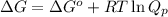
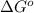 = Standard Gibbs free energy = -69.0 kJ/mol = -69000 J/mol (Conversion factor: 1 kJ = 1000 J)
= Standard Gibbs free energy = -69.0 kJ/mol = -69000 J/mol (Conversion factor: 1 kJ = 1000 J)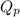 = Ratio of partial pressure of products and reactants =
= Ratio of partial pressure of products and reactants =