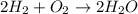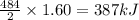Chemistry, 31.03.2020 01:21, LtotheJ0225
Consider the reaction 2 H 2 + O 2 ⟶ 2 H 2 O Δ H rxn = − 484 kJ Which answer best describes the transfer of heat that occurs when 1.60 mol H 2 reacts? 387 kJ absorbed 484 kJ released 387 kJ released 774 kJ absorbed 484 kJ absorbed 774 kJ released

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:30, michellealvarez985
What phase of matter has particles that are held together but can flow past each other and takes the shape of a container, filling it from the bottom up?
Answers: 1


Chemistry, 22.06.2019 15:30, sanchez7489
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1

Chemistry, 23.06.2019 08:00, hardwick744
How does the digestive system interact with the circulatory system? a. messages sent as electrical impulses from the digestive system are transported throughout the body by the circulatory system. b. nutrients taken in and broken down by the digestive system are carried to various parts of the body by the circulatory system. c. nutrients and gases are absorbed by organs in the circulatory system. then, they are transported to all parts of the body by organs in the digestive system. d. oxygen and carbon dioxide are exchanged by organs in the digestive system, and the gases are carried to the rest of the body by the circulatory system.
Answers: 2
Do you know the correct answer?
Consider the reaction 2 H 2 + O 2 ⟶ 2 H 2 O Δ H rxn = − 484 kJ Which answer best describes the trans...
Questions in other subjects:

Biology, 07.01.2020 13:31




Mathematics, 07.01.2020 13:31

Biology, 07.01.2020 13:31




Mathematics, 07.01.2020 13:31


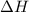 is positive.
is positive.