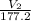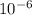Chemistry, 31.03.2020 01:01, victoria02117
Starting with a vessel of temperature 50.1°C, a pressure of 6263.MmHg and volume 461.1mL, what is its final volume, in liters, if you cool it to -95.8°C with a final pressure of 411.Atm?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:00, aschool2000
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 10:30, kluckey3426
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 10:30, tjjjjjjjjjjjjjjjjjjj
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1
Do you know the correct answer?
Starting with a vessel of temperature 50.1°C, a pressure of 6263.MmHg and volume 461.1mL, what is it...
Questions in other subjects:

Physics, 24.03.2020 08:47

Mathematics, 24.03.2020 08:47


Health, 24.03.2020 08:47


English, 24.03.2020 08:47

Geography, 24.03.2020 08:48


Biology, 24.03.2020 08:48

Social Studies, 24.03.2020 08:49


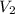 = 5.17 ml
= 5.17 ml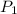 = 6263 mm Hg = 8.24 atm = 835 K pa
= 6263 mm Hg = 8.24 atm = 835 K pa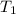 = 50.1 ° c = 323.1 K
= 50.1 ° c = 323.1 K = 461.1 ml = 0.0004611
= 461.1 ml = 0.0004611 
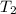 = - 95.8 ° c = 177.2 K
= - 95.8 ° c = 177.2 K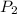 = 411 atm = 41644.6 K PA
= 411 atm = 41644.6 K PA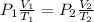
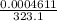 = 41644.6 ×
= 41644.6 ×