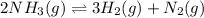Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, VictoriaRose520
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1

Chemistry, 22.06.2019 02:30, milesjreece3939
Select each correct answer. more than one answer may be correct. which of the following is a characteristic of unicellular organisms? they can possess tissues and organs. all of their functions are performed by a single cell. they are usually microscopic. each of their cells is specialized to perform a specific function.
Answers: 1

Chemistry, 22.06.2019 06:00, coolkid2041
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 20:40, larkinc2946
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1
Do you know the correct answer?
Consider the following system at equilibrium at 723 K: 2 NH3 (g) 26.6 kcal N2 (g) 3 H2 (g) Indicate...
Questions in other subjects:


Mathematics, 04.02.2021 07:20

Mathematics, 04.02.2021 07:20


Mathematics, 04.02.2021 07:20


Geography, 04.02.2021 07:20

Biology, 04.02.2021 07:20