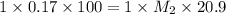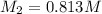Chemistry, 31.03.2020 01:05, bjpvrpow74wq
To standardize a solution of NaOH before using it in a titration of an unknown acid, you dissolve 3.56 grams of potassium hydrogen phthalate (KHP) into 100 mL of H2O. You then titrate the KHP with your sodium hydroxide solution and reach the endpoint after adding 20.9 mL of NaOH. What is the molarity of your sodium hydroxide solution? (Molecular Mass of KHP = 204.22 g/mol)

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 07:00, coolkid2041
Calculate the number of moles of ethane in 100 grams
Answers: 3

Chemistry, 22.06.2019 09:00, tashaunalewis4786
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1
Do you know the correct answer?
To standardize a solution of NaOH before using it in a titration of an unknown acid, you dissolve 3....
Questions in other subjects:



Mathematics, 24.05.2020 16:57

Mathematics, 24.05.2020 16:57


History, 24.05.2020 16:57

History, 24.05.2020 16:57

Mathematics, 24.05.2020 16:57


History, 24.05.2020 16:57


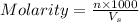
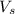 = volume of solution in ml = 100 ml
= volume of solution in ml = 100 ml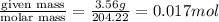
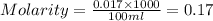
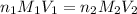
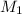 = molarity of KHP solution = 0.17 M
= molarity of KHP solution = 0.17 M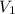 = volume of KHP solution = 100 ml
= volume of KHP solution = 100 ml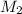 = molarity of NaOH solution = ?
= molarity of NaOH solution = ?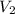 = volume of NaOH solution = 20.9 ml
= volume of NaOH solution = 20.9 ml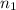 = valency of KHP = 1
= valency of KHP = 1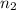 = valency of NaOH = 1
= valency of NaOH = 1