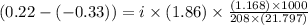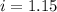Chemistry, 31.03.2020 01:05, raishagibson
Assume that you were assigned BaCl2 in lab. The water in your test tube weighed 21.797 g. Following the procedure in the lab manual, you determined that freezing point of water is 0.02oC. You weighed out 1.168 g of salt and added it to the original test tube, then determined that the freezing point was -0.33oC. Based on these experimental parameters, calculate the van't Hoff factor for BaCl2.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:10, josephpezza18
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 09:30, kevinh2683
Apump contains 0.5 l of air at 203 kpa. you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 11:00, 21villalobosjabez
Which type of fossil does this image depict?
Answers: 1

Chemistry, 23.06.2019 00:50, lakhanir2013
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3
Do you know the correct answer?
Assume that you were assigned BaCl2 in lab. The water in your test tube weighed 21.797 g. Following...
Questions in other subjects:


Mathematics, 25.01.2021 21:10




English, 25.01.2021 21:10



Computers and Technology, 25.01.2021 21:10


 is 1.15
is 1.15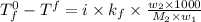
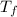 = freezing point of solution =
= freezing point of solution = 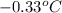
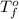 = freezing point of water =
= freezing point of water = 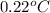
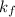 = freezing point constant of water =
= freezing point constant of water = 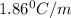
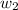 = mass of solute = 1.168 g
= mass of solute = 1.168 g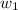 = mass of solvent (water) = 21.797 g
= mass of solvent (water) = 21.797 g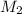 = molar mass of solute = 208 g/mol
= molar mass of solute = 208 g/mol