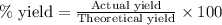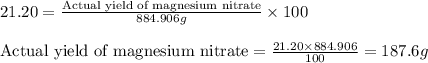Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, adrian08022
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2

Chemistry, 22.06.2019 10:30, jahmira96
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 14:30, Hannahmiller3773
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3

Chemistry, 22.06.2019 15:00, hockeykid7583
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2
Do you know the correct answer?
Assuming an efficiency of 21.20 % , calculate the actual yield of magnesium nitrate formed from 143....
Questions in other subjects:


Mathematics, 26.07.2019 18:00





History, 26.07.2019 18:00




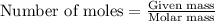 .....(1)
.....(1)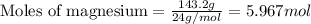
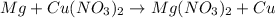
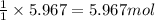 of magnesium nitrate
of magnesium nitrate