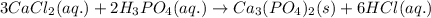Chemistry, 31.03.2020 01:03, thatcurlysophia
An aqueous solution of calcium chloride is added to aqueous phosphoric acid, and a white precipitate forms. Write the balanced chemical equation for this reaction. Phases are optional. Do not write an ionic equation (i. e., the answer should not show any charges).

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:30, TaraC
Read the claim. breakfast is an important meal. it jump starts the body’s process of using calories to break down food. appetite can decrease with age, but going too long without eating causes metabolism to slow down. current research shows that incorporating legumes such as lentils and chickpeas into meals boosts metabolism for twenty-four hours. who might benefit from this claim? people who have a fast metabolism stores that sell exercise equipment people who take vitamin supplements grocery stores that sell legumes
Answers: 1

Chemistry, 23.06.2019 10:00, Jennifer16253
What is the mass in grams of 12.26 ml of acetone
Answers: 1

Chemistry, 23.06.2019 12:50, ttangelique
What is the relative mass of an electron? a) 1/1840 the mass of a neutron + proton b) 1/1840 the mass of an alpha particle c) 1/1840 the mass of a c-12 atom d) 1/1840 the mass of a hydrogen atom
Answers: 3

Chemistry, 23.06.2019 13:00, madelyngv97
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1
Do you know the correct answer?
An aqueous solution of calcium chloride is added to aqueous phosphoric acid, and a white precipitate...
Questions in other subjects:

Mathematics, 21.05.2021 19:20


Mathematics, 21.05.2021 19:20

Mathematics, 21.05.2021 19:20


Mathematics, 21.05.2021 19:20

Mathematics, 21.05.2021 19:20

Social Studies, 21.05.2021 19:20


Chemistry, 21.05.2021 19:20