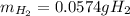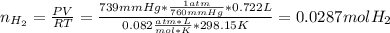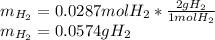Chemistry, 31.03.2020 00:45, emilyturchon
A sample that contains aluminum is reacted with sulfuric acid according to the equation given below. When the aluminum dissolves the total volume of gas collected over water at 25 °C is 0.722 L at a total pressure of 739 mm Hg. What mass of hydrogen gas is collected?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, Cartucho1978
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 22.06.2019 15:00, hockeykid7583
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2


Chemistry, 23.06.2019 02:30, roseemariehunter12
Asubstance is held in an open container. its particles move past one another at random speeds but do not leave the container. heat is removed from the system, and the particles slow down. when enough heat is removed, the particles no longer have enough speed to overcome the weak attractive forces between them. when this happens, the substance enters its solid state. the process described above is known as .
Answers: 3
Do you know the correct answer?
A sample that contains aluminum is reacted with sulfuric acid according to the equation given below....
Questions in other subjects:

Biology, 21.04.2021 15:50

Mathematics, 21.04.2021 15:50

Mathematics, 21.04.2021 15:50

Mathematics, 21.04.2021 15:50


Biology, 21.04.2021 15:50

Mathematics, 21.04.2021 15:50


English, 21.04.2021 15:50

English, 21.04.2021 15:50