

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:30, kittybatch345
Is a chemical message sent by another individual.
Answers: 1

Chemistry, 22.06.2019 23:30, emmalado45
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1


Chemistry, 23.06.2019 09:30, tramqpham25
People who practice which of the following diets may run the risk of not getting enough iron. a. gluten free or vegan diet b. diet for managing diabetes c. vegan diet d. gluten free diet
Answers: 2
Do you know the correct answer?
If you combine 250.0 mL 250.0 mL of water at 25.00 ∘ C 25.00 ∘C and 100.0 mL 100.0 mL of water at 95...
Questions in other subjects:

Health, 18.03.2021 01:50





Computers and Technology, 18.03.2021 01:50


Physics, 18.03.2021 01:50

Chemistry, 18.03.2021 01:50

Mathematics, 18.03.2021 01:50


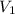 = 250 ml
= 250 ml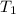 = 25 °c
= 25 °c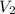 = 100 ml
= 100 ml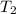 = 95 °c
= 95 °c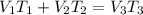
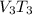
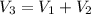
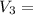 250 + 100 = 350 ml
250 + 100 = 350 ml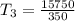
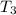 = 45 °c
= 45 °c



