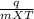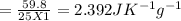Chemistry, 31.03.2020 00:53, amanda2517
You did an experiment in which you found that 59.8 J was required to raise the temperature of 25.0 g of ethylene glycol ( a compound used as antifreeze in automobile engines) by 1.00 Kelvin. Calculate the specific heat capacity of ethylene glycol from these data

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:30, candigirl8847
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 13:30, bryce99
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 15:00, Zagorodniypolina5
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2
Do you know the correct answer?
You did an experiment in which you found that 59.8 J was required to raise the temperature of 25.0 g...
Questions in other subjects:

Arts, 02.05.2021 01:00


Mathematics, 02.05.2021 01:00


Mathematics, 02.05.2021 01:00


Chemistry, 02.05.2021 01:00

Medicine, 02.05.2021 01:00


History, 02.05.2021 01:00