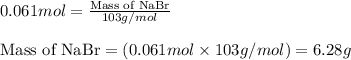Chemistry, 31.03.2020 00:27, coltonwsmith
Aqueous hydrobromic acid will react with solid sodium hydroxide to produce aqueous sodium bromide and liquid water . Suppose 17. g of hydrobromic acid is mixed with 2.44 g of sodium hydroxide. Calculate the maximum mass of sodium bromide that could be produced by the chemical reaction. Be sure your answer has the correct number of significant digits.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:20, emilyborland50
Which of the following statements is false regarding aromaticity? a. the compound must be cyclic b. the compound must be fully conjugated c. the compound must be planar d. the number of electrons in the pi system must satisfy the hückel 4n+2 rule e. the compound must have a neutral charge
Answers: 2


Chemistry, 22.06.2019 06:30, jonloya264
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 15:00, emmalie52
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1
Do you know the correct answer?
Aqueous hydrobromic acid will react with solid sodium hydroxide to produce aqueous sodium bromide an...
Questions in other subjects:



Mathematics, 16.03.2020 21:26

Mathematics, 16.03.2020 21:26




Mathematics, 16.03.2020 21:26

Mathematics, 16.03.2020 21:26


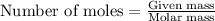 .....(1)
.....(1)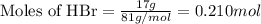
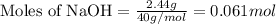

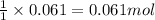 of HBr
of HBr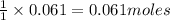 of carbon dioxide
of carbon dioxide