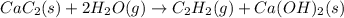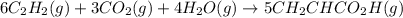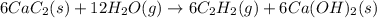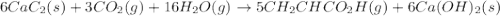Chemistry, 31.03.2020 00:21, isabelperez063
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. In the first step, calcium carbide and water react to form acetylene and calcium hydroxide: (s)(g)(g)(s) In the second step, acetylene, carbon dioxide and water react to form acrylic acid: (g)(g)(g)(g) Write the net chemical equation for the production of acrylic acid from calcium carbide, water and carbon dioxide. Be sure your equation is balanced.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 00:00, familyk0jj3
How do you determine the percent yield of a chemical reaction
Answers: 1

Chemistry, 23.06.2019 03:00, rhianna18
In november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 1

Do you know the correct answer?
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of...
Questions in other subjects:

Mathematics, 28.04.2021 18:40



Mathematics, 28.04.2021 18:40

Mathematics, 28.04.2021 18:40