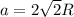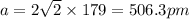Chemistry, 31.03.2020 00:23, tartcandi303
A certain metal M crystallizes in a lattice described by a face-centered cubic (fcc) unit cell. The radius r of M atoms has been measured to be 179.pm. Calculate the lattice constant a of a crystal of M. Be sure your answer has the correct number of significant digits, and be sure it has the correct unit symbol.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:40, loveoneonly9153
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1


Chemistry, 22.06.2019 13:30, xojade
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1
Do you know the correct answer?
A certain metal M crystallizes in a lattice described by a face-centered cubic (fcc) unit cell. The...
Questions in other subjects:

Chemistry, 04.07.2019 15:00

History, 04.07.2019 15:00







Mathematics, 04.07.2019 15:00

Health, 04.07.2019 15:00