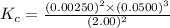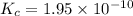Chemistry, 30.03.2020 23:54, dannaasc5475
1 Potassium chlorate decomposes to product potassium chloride and oxygen gas. 2KClO3(s) ⇔ 2KCl(s) + 3O2(g) When this reaction was run at room temperature, the following equilbrium concentrations were measured: [O2] = 0.0500 M; [KCl] = 0.00250 M; [KClO3] = 2.00 M What is the equilibrium constant for this reaction?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:40, CylieTbh
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3



Chemistry, 22.06.2019 11:40, jerrysandoval22
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2
Do you know the correct answer?
1 Potassium chlorate decomposes to product potassium chloride and oxygen gas. 2KClO3(s) ⇔ 2KCl(s) +...
Questions in other subjects:

Social Studies, 21.08.2019 22:30

Computers and Technology, 21.08.2019 22:30




Mathematics, 21.08.2019 22:30




History, 21.08.2019 22:30


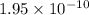
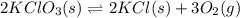
![K_c=\frac{[KCl]^2\times [O_2]^3}{[KClO_3]^2}](/tpl/images/0571/9600/641b5.png)