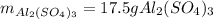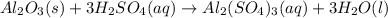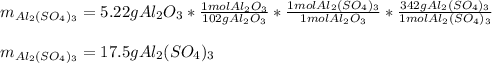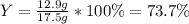Chemistry, 30.03.2020 23:55, guadalupemarlene2001
For the following reaction, 5.22 grams of aluminum oxide are mixed with excess sulfuric acid. The reaction yields 12.9 grams of aluminum sulfate. aluminum oxide (s) + sulfuric acid (aq) aluminum sulfate (aq) + water (l) What is the theoretical yield of aluminum sulfate ?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:00, youngchapo813p8d9u1
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

Chemistry, 22.06.2019 12:00, 1963038660
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1

Chemistry, 22.06.2019 20:30, dinapaul424
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1
Do you know the correct answer?
For the following reaction, 5.22 grams of aluminum oxide are mixed with excess sulfuric acid. The re...
Questions in other subjects:



Biology, 30.10.2020 14:00




English, 30.10.2020 14:00

Mathematics, 30.10.2020 14:00

History, 30.10.2020 14:00

Mathematics, 30.10.2020 14:00