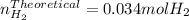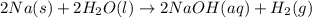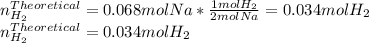Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, heavyhearttim
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 05:00, adrian128383
What forms when chemical reactions combine pollution with sunlight?
Answers: 1


Chemistry, 22.06.2019 10:30, angemango3423
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1
Do you know the correct answer?
When sodium metal is added to water, the following reaction occurs: 2Na(s) + 2H2O(l) → 2NaOH(aq) + H...
Questions in other subjects:

History, 11.10.2019 13:30

World Languages, 11.10.2019 13:30

Social Studies, 11.10.2019 13:30



Health, 11.10.2019 13:30



English, 11.10.2019 13:30