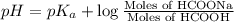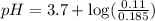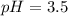Chemistry, 30.03.2020 23:33, erikamaldonado661
Calculate the pH of a solution that contains 0.250 M formic acid, HCOOH (Ka =1.8 x 10-4 ), and 0.100M sodium formate, HCOONa after the addition of 10.0 mL of 6.00M NaOH to the original buffered solution volume of 500.0 mL.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:30, cadenhuggins2
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 10:30, cheyennecarrillo14
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Do you know the correct answer?
Calculate the pH of a solution that contains 0.250 M formic acid, HCOOH (Ka =1.8 x 10-4 ), and 0.100...
Questions in other subjects:


Computers and Technology, 26.07.2019 15:30

Physics, 26.07.2019 15:30


Chemistry, 26.07.2019 15:30


Mathematics, 26.07.2019 15:30

Mathematics, 26.07.2019 15:30



 ,
, 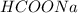 and
and 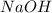 .
.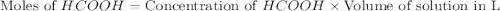
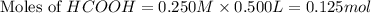
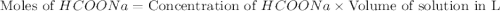
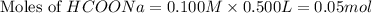
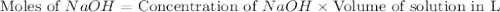
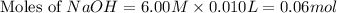
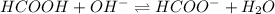
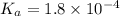
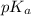 .
.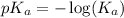
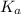 in this expression, we get:
in this expression, we get: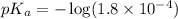
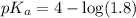
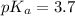
![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0571/8655/e961a.png)
![pH=pK_a+\log \frac{[HCOONa]}{[HCOOH]}](/tpl/images/0571/8655/c5edc.png)
![pH=pK_a+\log \frac{[\frac{\text{Moles of HCOONa}}{\text{Volume of solution}}]}{[\frac{\text{Moles of HCOOH}}{\text{Volume of solution}}]}](/tpl/images/0571/8655/fba20.png)