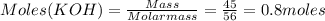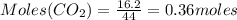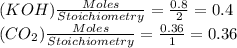For the following reaction, 16.2 grams of carbon dioxide are allowed to react with 45.0 grams of potassium hydroxide. carbon dioxide (g) potassium hydroxide (aq) potassium carbonate (aq) water (l) What is the maximum amount of potassium carbonate that can be formed

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, noathequeen
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 08:30, itzhari101
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 22.06.2019 09:10, GreatBaconGamer
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Do you know the correct answer?
For the following reaction, 16.2 grams of carbon dioxide are allowed to react with 45.0 grams of pot...
Questions in other subjects:







Biology, 20.03.2020 11:11