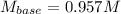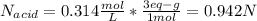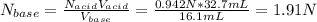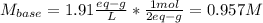Chemistry, 30.03.2020 23:35, ethanyayger
A 16.1 mL sample of Ba(OH)2 is titrated with H3PO4. If 32.7 mL of 0.314 M H3PO4 is needed to reach the endpoint, what is the concentration (M) of the Ba(OH)2 solution? 3 Ba(OH)2(aq) + 2 H3PO4(aq) → Ba3(PO4)2(aq) + 6 H2O(l)

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, carlybeavers50
The graph above shows how the price of cell phones varies with the demand quantity. the equilibrium price for cell phones is where both supply and demand quantities equal $100, 5,000 5,000, $100
Answers: 2

Chemistry, 22.06.2019 06:30, reecedstceklein
Over the last 90 years, scientists have added to the body of evidence supporting the big bang theory. what is the latest piece of evidence discovered in 2014?
Answers: 1

Chemistry, 23.06.2019 13:30, jcastronakaya
The zinc within a copper-plated penny dissolves in hydrochloric acid if the copper coating is filed down in several spots (so that the hydrochloric acid can reach the zinc). the reaction between the acid and the zinc 2h+(aq)+zn(s)→h2(g)+zn2+(aq) . when the zinc in a certain penny dissolves, the total volume of gas collected over water at 25 °c is 0.947 l at a total pressure of 743 mmhg . (vapor pressure of water is 23.78 mmhg at 25 °c .) what mass of hydrogen gas is collected? answer in appropriate significant figures
Answers: 3

Chemistry, 23.06.2019 18:00, coopyishome
Which of the following hydrocarbons will most likely be found around cows? a. ethane b. methane c. octane d. decane
Answers: 2
Do you know the correct answer?
A 16.1 mL sample of Ba(OH)2 is titrated with H3PO4. If 32.7 mL of 0.314 M H3PO4 is needed to reach t...
Questions in other subjects:



History, 06.11.2019 18:31





Mathematics, 06.11.2019 18:31


History, 06.11.2019 18:31