
Chemistry, 30.03.2020 23:26, Whitehouse9
The Michaelis‑Menten equation models the hyperbolic relationship between [S] and the initial reaction rate V 0 V0 for an enzyme‑catalyzed, single‑substrate reaction E + S − ⇀ ↽ − ES ⟶ E + P E+S↽−−⇀ES⟶E+P . The model can be more readily understood when comparing three conditions:
[ S ] < [ S ]>>Km
[ S ] = K m
Match each statement with the condition that it describes. Note that "rate" refers to initial velocity V 0 where steady state conditions are assumed. [ E total ] refers to the total enzyme concentration and [Efree] refers to the concentration of free enzyme.
1. [ S ] < 2. [ S ]>>Km
3. [ S ] = K m
4. Not true for any of these conditions.
A. [Efree] is about equal to [Etotal]
B. Half of the active sites are filled with S.
C. [ES] is much lower than [Efree]
D. Almost all active sites will be filled.
E. Increasing [Etotal] will lower Km.
F. Reaction rate is independent of [S]

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:00, anaalashay
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 18:00, liddopiink1
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3

Chemistry, 22.06.2019 18:20, juansebas35
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2
Do you know the correct answer?
The Michaelis‑Menten equation models the hyperbolic relationship between [S] and the initial reactio...
Questions in other subjects:











![V_0 = \dfrac{K_{cat} \times [E_0] \times [S] }{\left(K_m+[S] \right)}](/tpl/images/0571/8305/b22e2.png)
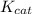 = The experimental reaction rate
= The experimental reaction rate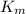 = The Michaelis-Menten constant
= The Michaelis-Menten constant![V_0 = \dfrac{K_{cat} \times [E_0] \times [S] }{\left(K_m \right)}](/tpl/images/0571/8305/b4b90.png)
![[E_{total}]](/tpl/images/0571/8305/eeaac.png) =
= ![[E_0]](/tpl/images/0571/8305/5356d.png) =
= ![[E_{free}]](/tpl/images/0571/8305/6dbaa.png) + [ES]
+ [ES]![V_0 = \dfrac{K_{cat} \times [E_{total}] \times [S] }{\left([S]+[S] \right)} = \dfrac{1}{2} \times K_{cat} \times [E_{total}]](/tpl/images/0571/8305/aac62.png)
![V_0 = \dfrac{K_{cat} \times [E_0] \times [S] }{\left([S] \right)} = K_{cat} \times [E_0]](/tpl/images/0571/8305/605c5.png)




