
Chemistry, 30.03.2020 23:13, slonekaitlyn01
Consider a galvanic cell in which Al 3 is reduced to elemental aluminum and magnesium metal is oxidized to Mg 2 . Write the balanced half-cell reactions that take place at the cathode and at the anode.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:40, jaueuxsn
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2

Chemistry, 22.06.2019 09:30, raizagisselle1694
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3


Chemistry, 22.06.2019 19:40, trodgers0202
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests. which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3
Do you know the correct answer?
Consider a galvanic cell in which Al 3 is reduced to elemental aluminum and magnesium metal is oxidi...
Questions in other subjects:





English, 05.11.2019 20:31

Biology, 05.11.2019 20:31

Mathematics, 05.11.2019 20:31


Biology, 05.11.2019 20:31


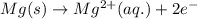 ( × 3)
( × 3)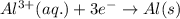 ( × 2)
( × 2)




