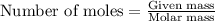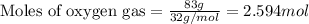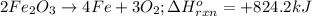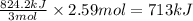Chemistry, 30.03.2020 23:14, constipatedcow18
Consider the reaction:
2Fe2O3⟶4Fe+3O2 ΔH∘rxn=+824.2 kJ
The formation of 83.0 g of O2 results in:
A. the absorption of 22800 kJ of heat.
B. the release of 2140 kJ of heat.
C. the absorption of 2140 kJ of heat.
D. the release of 713 kJ of heat.
E. the absorption of 713 kJ of heat.
F. the release of 22800 kJ of heat.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, hdjsjfjruejchhehd
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1



Chemistry, 23.06.2019 03:00, BeeShyanne
Use the half-reactions of the reaction au(oh)3 + hi -> au +i2 +h2o to answer the questions
Answers: 1
Do you know the correct answer?
Consider the reaction:
2Fe2O3⟶4Fe+3O2 ΔH∘rxn=+824.2 kJ
The formation of 83.0 g of...
2Fe2O3⟶4Fe+3O2 ΔH∘rxn=+824.2 kJ
The formation of 83.0 g of...
Questions in other subjects:




Mathematics, 02.02.2020 19:48

Social Studies, 02.02.2020 19:48

Mathematics, 02.02.2020 19:48

English, 02.02.2020 19:48

Mathematics, 02.02.2020 19:48


English, 02.02.2020 19:48