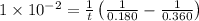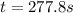Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, mimireds5419
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3

Chemistry, 22.06.2019 16:40, roderickhinton
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

Chemistry, 22.06.2019 23:00, Mw3spartan17
What extra step distinguishes fermentation from glycolysis
Answers: 1

Chemistry, 23.06.2019 00:20, cmflores3245
4. propanol and isopropanol are isomers. this means that they have a) the same molecular formula but different chemical properties. b) different molecular formulas but the same chemical properties. c) the same molecular formula and the same chemical properties. d) the same molecular formula but represent different states of the compound
Answers: 3
Do you know the correct answer?
A reaction that is second-order in one reactant has a rate constant of 1 × 10–2 L/mol · s. If the in...
Questions in other subjects:



Mathematics, 05.12.2020 05:50


Biology, 05.12.2020 05:50





Mathematics, 05.12.2020 05:50


![k=\frac{1}{t}\left (\frac{1}{[A]}-\frac{1}{[A]_o}\right)](/tpl/images/0571/7035/5ea71.png)
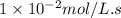
![[A]_o](/tpl/images/0571/7035/9caf5.png) = Initial concentration = 0.360 mol/L
= Initial concentration = 0.360 mol/L