
Chemistry, 30.03.2020 23:11, pedroramirezr2
130.0 grams of NaNO 3 is dissolved in water to make a 5.00 L solution. What is the molarity of the solution? (Molar mass of NaNo3 =g/mol) what is the molarity

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, DarcieMATHlin2589
Write a brief passage describing a neutral atom of nitrogen-14 (n-14). describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. use the periodic table to you. 14 protons and eletrons since its a neutral atom
Answers: 1

Chemistry, 22.06.2019 15:30, dylannhandy
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 23.06.2019 03:30, nikkio4
In chemistry, the type of an atom (what element it is) is determined by: a) the number of protons it contains in its nucleus. b) the number of neutrons it contains in its nucleus. c) the number of protons it has in a cloud around the nucleus. d) the number of neutrons it has in a cloud around the nucleus. e) the number of electrons it exchanges with its neighbors.
Answers: 1

Chemistry, 23.06.2019 09:00, rebeccathecatt
Which of the following are in a chemical family a. ca, sc, k b. cu, ag, au c. so, ge, sb
Answers: 1
Do you know the correct answer?
130.0 grams of NaNO 3 is dissolved in water to make a 5.00 L solution. What is the molarity of the s...
Questions in other subjects:

Mathematics, 05.11.2019 00:31

Mathematics, 05.11.2019 00:31



Social Studies, 05.11.2019 00:31


History, 05.11.2019 00:31



Mathematics, 05.11.2019 00:31


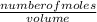
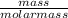 =
= 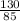 = 1.53moles
= 1.53moles 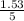 = 0.31M
= 0.31M



