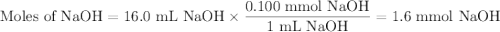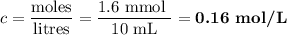Chemistry, 30.03.2020 22:52, gghkooo1987
Base your answer on the information below and on your knowledge of chemistry.
A NaOH(aq) solution with a pH value of 13 is used to determine the molarity of an HCl(aq) solution. A 10.0-mL sample of the HCl(aq) is exactly neutralized by 16.0 mL of 0.100 M NaOH(aq). During this laboratory activity, appropriate safety equipment was used and safety procedures were followed.
Determine the pH value of a solution that has a H+(aq) ion concentration 10 times greater than the original NaOH(aq) solution.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:10, board1692
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3

Chemistry, 23.06.2019 01:30, jonmorton159
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1
Do you know the correct answer?
Base your answer on the information below and on your knowledge of chemistry.
A NaOH(aq)...
A NaOH(aq)...
Questions in other subjects:


Mathematics, 02.09.2019 08:30







History, 02.09.2019 08:30

Social Studies, 02.09.2019 08:30