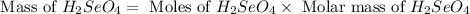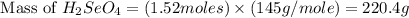Chemistry, 30.03.2020 22:32, savannahvargas512
Determine the limiting reactant in a mixture containing 139 g of Se, 431 g of Cl2, and 110 g of H2O. Calculate the maximum mass (in grams) of selenic acid, H2SeO4, that can be produced in the reaction.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, giraffegurl
If 34.2 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 299 kelvin and 1.21 atmospheres? 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 09:00, oliviacolaizzi
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 21:00, lalaween098
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3

Chemistry, 22.06.2019 23:00, chastineondre7979
What is the formula of the ionic compound composed of calcium cations and chloride anions
Answers: 1
Do you know the correct answer?
Determine the limiting reactant in a mixture containing 139 g of Se, 431 g of Cl2, and 110 g of H2O....
Questions in other subjects:



History, 04.12.2020 23:40







English, 04.12.2020 23:40


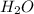
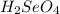 produced is, 220.4 grams.
produced is, 220.4 grams.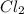 = 431 g
= 431 g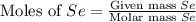
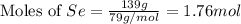
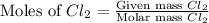
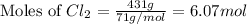
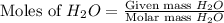
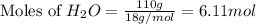
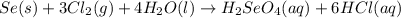
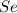
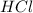 react with
react with 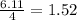 moles of
moles of 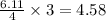 moles of
moles of 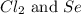 are excess reagent because the given moles are greater than the required moles and
are excess reagent because the given moles are greater than the required moles and