Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, Bradgarner772
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1

Chemistry, 22.06.2019 10:30, freddhendrickss
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 12:30, nekathadon
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 13:00, aleilyg2005
If two objects at different te, peraure are in contact with each other what happens to their temperature
Answers: 1
Do you know the correct answer?
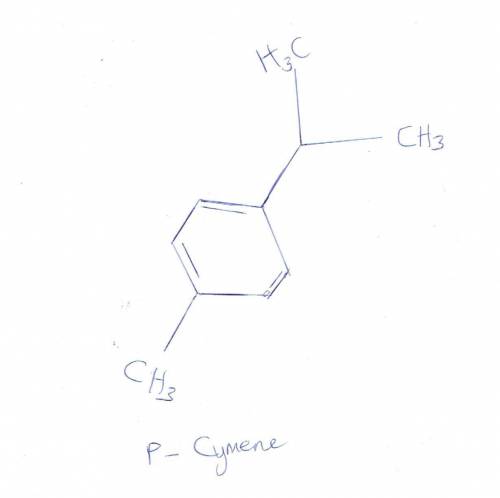
A compound, C10H14, shows an IR peak at 825 cm-1. Its 1H NMR spectrum has peaks at delta 7.0 (4 H, b...
Questions in other subjects:

Social Studies, 20.02.2020 03:13

Social Studies, 20.02.2020 03:13


Mathematics, 20.02.2020 03:13

Business, 20.02.2020 03:13