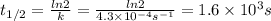Chemistry, 30.03.2020 21:50, karenjjensen
The rate constant, k, for a first-order reaction is equal to 4.3 × 10-4 s-1. What is the half-life for the reaction? The rate constant, k, for a first-order reaction is equal to 4.3 × 10-4 s-1. What is the half-life for the reaction? 1.9 × 103 s 1.2 × 103 s 1.6 × 103 s 3.0 × 10-4 s

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, nique0808
What are the major products produced in the combustion of c10h22 under the following conditions? write balanced chemical equations for each. a. an excess of oxygen b. a slightly limited oxygen supply c. a very limited supply of oxygen d. the compound is burned in air
Answers: 2



Chemistry, 22.06.2019 12:30, kaliyab191
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2
Do you know the correct answer?
The rate constant, k, for a first-order reaction is equal to 4.3 × 10-4 s-1. What is the half-life f...
Questions in other subjects:

Physics, 03.03.2021 21:20



History, 03.03.2021 21:20

English, 03.03.2021 21:20




Mathematics, 03.03.2021 21:20


![rate=k \times [A]^{m}](/tpl/images/0571/4200/f39c7.png)