
Chemistry, 30.03.2020 21:46, deanazilyiah
A major component of gasoline is octane . When octane is burned in air, it chemically reacts with oxygen gas to produce carbon dioxide and water . What mass of "oxygen gas is consumed" by the reaction of 6.1 g of octane

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, giraffegurl
If 34.2 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 299 kelvin and 1.21 atmospheres? 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 04:00, armahoney8566
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 22.06.2019 06:30, dpchill5232
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 08:00, tchase0616
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1
Do you know the correct answer?
A major component of gasoline is octane . When octane is burned in air, it chemically reacts with ox...
Questions in other subjects:

English, 07.11.2020 02:30


Mathematics, 07.11.2020 02:30

Physics, 07.11.2020 02:30

Mathematics, 07.11.2020 02:30

Geography, 07.11.2020 02:30

Biology, 07.11.2020 02:30

Business, 07.11.2020 02:30

English, 07.11.2020 02:30

Social Studies, 07.11.2020 02:30


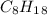 and its molecular mass is 114.23 g/mol.
and its molecular mass is 114.23 g/mol.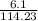 = 0.053 moles
= 0.053 moles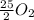 =
= 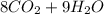
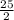 moles of oxygen.
moles of oxygen.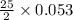 = 0.663 moles oxygen
= 0.663 moles oxygen



