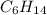Chemistry, 30.03.2020 21:23, jaleelbrown80
When 7.085 grams of a hydrocarbon, CxHy, were burned in a combustion analysis apparatus, 21.71 grams of CO2 and 10.37 grams of H2O were produced. In a separate experiment, the molar mass of the compound was found to be 86.18 g/mol. Determine the empirical formula and the molecular formula of the hydrocarbon.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, dpazmembreno
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 23.06.2019 05:40, shelbylynn1093
Why is it incorrect to balance a chemical equation by changing the subscripts? explain.
Answers: 2


Do you know the correct answer?
When 7.085 grams of a hydrocarbon, CxHy, were burned in a combustion analysis apparatus, 21.71 grams...
Questions in other subjects:



Geography, 22.08.2019 11:00


Mathematics, 22.08.2019 11:00

Mathematics, 22.08.2019 11:00

Mathematics, 22.08.2019 11:00

Social Studies, 22.08.2019 11:00

Mathematics, 22.08.2019 11:00

Mathematics, 22.08.2019 11:00