
Chemistry, 30.03.2020 21:22, dpazmembreno
A 35.0 L sample of gas collected in the upper atmosphere at a pressure of 48.6 torr is compressed into a 150. mL container at constant temperature. What is the new pressure (atm)? (4pts) 1 atm = 760 torr

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 19:30, gracieisweird12
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 23.06.2019 00:50, alainacorkell6472
What is the enthalpy of combustion (per mole) of c4h10 (g)? –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol
Answers: 1

Chemistry, 23.06.2019 13:00, Crxymia
How long could you survive without electricity? what parts of your life would be affected by loss of electricity? should you prepare for an electricity outage, and if so, how would you prepare? what backup system could your family or community install to generate limited amounts of electricity during an outage? how does this system create an electric force field and generate electric current?
Answers: 2
Do you know the correct answer?
A 35.0 L sample of gas collected in the upper atmosphere at a pressure of 48.6 torr is compressed in...
Questions in other subjects:



Mathematics, 29.04.2021 05:10



Biology, 29.04.2021 05:10

History, 29.04.2021 05:10

History, 29.04.2021 05:10


Mathematics, 29.04.2021 05:10



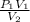
 or 11172 torr
or 11172 torr 



