
Chemistry, 30.03.2020 21:01, gungamer720
G Identify limiting reactants (mole ratio method). Close Problem Identify the limiting reactant in the reaction of bromine and chlorine to form BrCl, if 29.7 g of Br2 and 11.2 g of Cl2 are combined. Determine the amount (in grams) of excess reactant that remains after the reaction is complete.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, erinxmeow8
What are the charges of the subatomic particles by choosing the answer from the drop down menu. protons have a (+1,0,or-1). (protons, neutrons, electrons) have a 0 charge. 3.) electrons have a (+1,0,-1)
Answers: 2

Chemistry, 22.06.2019 02:50, JuniperGalaxy
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 05:00, foreignking02
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

Chemistry, 22.06.2019 09:00, alydiale584
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1
Do you know the correct answer?
G Identify limiting reactants (mole ratio method). Close Problem Identify the limiting reactant in t...
Questions in other subjects:

Mathematics, 22.09.2019 23:00

Mathematics, 22.09.2019 23:00

Physics, 22.09.2019 23:00



English, 22.09.2019 23:00

Social Studies, 22.09.2019 23:00

Mathematics, 22.09.2019 23:00


Social Studies, 22.09.2019 23:00


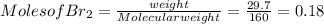
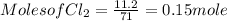
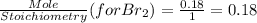
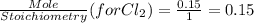
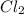 is a limiting reactant and Br₂ is excess reactant.
is a limiting reactant and Br₂ is excess reactant.



