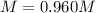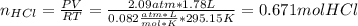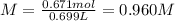Chemistry, 30.03.2020 21:05, jailenevazquez755
A 1.78−L sample of hydrogen chloride (HCl) gas at 2.09 atm and 22°C is completely dissolved in 699 mL of water to form hydrochloric acid solution. Calculate the molarity of the solution. Assume no change in volume.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, Dreynolds1667
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1


Chemistry, 22.06.2019 22:00, aliciaa101
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1

Chemistry, 23.06.2019 05:40, 19youngr
Which order shows the levels of organization from largest to smallest? organism, organ system, cell, organ, tissue organism, tissue, organ system, organ, cell organism, organ, organ system, cell, tissue organism, organ system, organ, tissue, cell
Answers: 2
Do you know the correct answer?
A 1.78−L sample of hydrogen chloride (HCl) gas at 2.09 atm and 22°C is completely dissolved in 699 m...
Questions in other subjects:



Chemistry, 27.01.2021 17:10


Mathematics, 27.01.2021 17:10


Mathematics, 27.01.2021 17:10