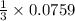Chemistry, 30.03.2020 21:19, ramzieboy13
Enter your answer in the provided box. Consider the reaction: N2(g) + 3H2(g) → 2NH3(g) Suppose that a particular moment during the reaction, molecular hydrogen is reacting with a magnitude of 0.0759 M/s. At what rate is molecular nitrogen reacting?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:00, IsabellaGracie
True or false, the three major scales used to measure earthquakes are mercalli scale, richter scale and magnitude scale
Answers: 2

Chemistry, 22.06.2019 02:50, Jerrikasmith28
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Do you know the correct answer?
Enter your answer in the provided box. Consider the reaction: N2(g) + 3H2(g) → 2NH3(g) Suppose that...
Questions in other subjects:


Mathematics, 25.05.2021 23:40


Mathematics, 25.05.2021 23:40

Computers and Technology, 25.05.2021 23:40




English, 25.05.2021 23:40

Mathematics, 25.05.2021 23:40


![\frac{d[N_2]}{dt}](/tpl/images/0571/2684/73dfe.png) = -
= - ![\frac{1}{3} \frac{d[H_2]}{dt}](/tpl/images/0571/2684/8a6b8.png) = +
= + ![\frac{1}{2} \frac{d[NH_3]}{dt}](/tpl/images/0571/2684/073e6.png)