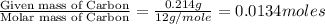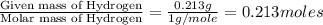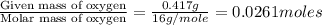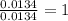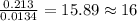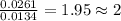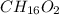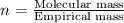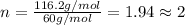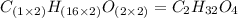Chemistry, 30.03.2020 21:13, robertjoy19
The smell of dirty gym socks is caused by the compound caproic acid(contains H, C, O). Combustion of 0.844 g of caproic acid produced 0.784 g of H2O and 1.92 g of CO2. If the molar mass of caproic acid is 116.2 g/mol, what is the molecular formula of caproic acid? (MM C = 12g/mol, H = 1g/mol, O = 16 g/mol)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, jetblackcap
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1

Chemistry, 22.06.2019 20:40, larkinc2946
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1

Chemistry, 22.06.2019 22:00, Porciabeauty6788
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2
Do you know the correct answer?
The smell of dirty gym socks is caused by the compound caproic acid(contains H, C, O). Combustion of...
Questions in other subjects:


Physics, 13.11.2021 06:30

English, 13.11.2021 06:30

Mathematics, 13.11.2021 06:30


Business, 13.11.2021 06:30



Mathematics, 13.11.2021 06:30


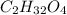
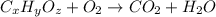
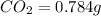
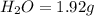
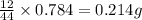 of carbon will be contained.
of carbon will be contained.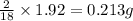 of hydrogen will be contained.
of hydrogen will be contained.