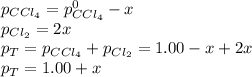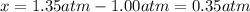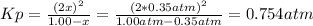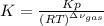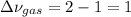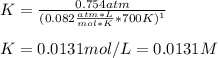Chemistry, 30.03.2020 20:36, Anaaguayo954
At elevated temperature, carbon tetrachloride decomposes to its elements: CCl4(g) C(s) 2Cl2(g). At 700 K, if the initial pressure of CCl4 is 1. 00 atm and at equilibrium the total pressure is 1. 35 atm, then calculate K

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:30, ashtonviceoxd21i
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b. colder climates near the equator c. large waves on the cost of europe d. warm climates in northern europe
Answers: 1


Chemistry, 23.06.2019 21:00, jmallory3031
What is the chemical fluoride for carbon tetrafluoride?
Answers: 1

Chemistry, 23.06.2019 23:20, CoolxBreeze
How much heat transfer (in kj) is necessary to raise the temperature of a 0.250 kg piece of ice from −24.0°c to 128°c, including the energy needed for phase changes? (assume the substance remains at a constant volume throughout each stage of the heating process.)
Answers: 3
Do you know the correct answer?
At elevated temperature, carbon tetrachloride decomposes to its elements: CCl4(g) C(s) 2Cl2(g). At 7...
Questions in other subjects:


Mathematics, 07.07.2019 10:00

Mathematics, 07.07.2019 10:00



English, 07.07.2019 10:00



English, 07.07.2019 10:00


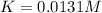
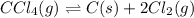
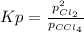
 due to reaction extent:
due to reaction extent: